Mastitis is a commonly encountered disease of suckling sheep. Veterinary surgeons will frequently be presented with severe, acute cases, or consulted by farmers about treatment and prevention. Acute mastitis can result in the death of the ewe, and both acute and chronic mastitis will result in the culling of the ewe (increasing replacement rate and reducing ewe longevity), and will negatively impact ewe welfare, milk production and lamb growth rate. It is likely that the impact of mild and subclinical mastitis in meat sheep is underestimated. The studies that have been performed indicate that these conditions can have a significant negative impact on lamb growth rate. It is important for practitioners to understand the aetiology, risk factors, treatment and prevention options in order to minimise the welfare and economic impacts of the disease.
Prevalence and economic impact
Mastitis is a problem encountered in all sheep flocks, although incidence can vary widely. Grant et al (2016), performed mammary examinations on 4721 ewes across 10 suckler flocks over a 2-year period. This was done at varying stages throughout the production year and recorded a prevalence of 2.1–3.0% cases of acute mastitis per year. Also in the study 4.7% of ewes had intramammary masses during pregnancy and 10.9% of ewes had intramammary masses during lactation. (Intramammary masses are associated with chronic mastitis and are a frequent reason for culling ewes out of the flock, regardless of their age or stage of productive life). (Figure 1).
The extent of subclinical and clinical mastitis in flocks may be under reported at farm level. Investigations where lamb growth rates and ewe condition are reduced or below target may often reveal cases of mastitis that have gone undetected. Watkins et al (1991) performed milk assessments of all sheep from seven flocks at 3-weekly intervals and reported an incidence of subclinical mastitis of 11.7%, and prevalence varying between 5.5 and 7.0%.
Acute cases of mastitis often lead to mortality. Post-mortem examination revealed mastitis to be responsible for 13/259 (5%) of ewe deaths in a study of 33 sentinel flocks in Ireland (Murray et al, 2019). Other direct and indirect costs arise from:
- Increased ewe mortality
- Increased replacement rate
- Decreased ewe longevity
- Decreased lamb growth rates
- Increased lamb mortality (directly as a result of starvation, and indirectly as underfed lambs are at greater risk of other diseases)
- Increased workload because of artificial rearing of lambs.
Currently there is no work to accurately estimate the direct costs associated with a case of mastitis per ewe. A model was produced using a study population of Texel ewes to assess breeding for resistance to mastitis among other control methods. This indicated that reducing the overall risk of mastitis by 10% could save £8.40 per ewe (Conington et al, 2008). In addition, in a study in New Zealand, ewes that had palpable udder abnormalities at pre-mating examination went on to rear 25.8–33.2% fewer kilograms of lamb in the following season than ewes with normal udder palpation (Flay et al, 2020).
In a postal questionnaire completed by 329 English sheep farmers, 94.2% of respondents did not retain ewes that have suffered from mastitis (Cooper et al, 2016). Increased mastitis incidence may therefore increase premature culling and cause the replacement rate to rise above the recommended target of 20–25% per year. This increase in the replacement rate will increase costs as a result of replacement purchase/rearing, and may also lower the average age of the flock, potentially leading to reduced reproductive performance (Farrell et al, 2019). While there are no studies directly assessing the economic impact of a mastitis-related increase in the replacement rate, modelling of an 1800-ewe hill country beef and sheep farm in New Zealand estimated that the cash operating surplus for the sheep enterprise would be reduced by NZ$ 1069 (£543/€636) per year per 1% increase in ewe wastage (mortality plus premature culling) (Farrell et al, 2019).
The impacts of subclinical mastitis are less clear. Fthenakis and Jones (1990) showed that subclinical mastitis significantly reduced ewe milk production and lamb growth rate, so some of the impact from clinical cases (see above) also holds true for subclinical mastitis.
Clinical signs
The clinical signs of mastitis in meat sheep vary depending on the severity of the mastitis.
Subclinical and mild mastitis (i.e. where there are changes to the milk composition only) are not likely to be detected by farmers.
In acute mastitis, detection of affected ewes may be as a result of hungry lambs, a stiff-legged gait of the ewe, or the ewe appearing unwell. On closer examination affected ewes may be dull, pyrexic, dehydrated, with injected or toxaemic mucous membranes (Figure 2). Rumen fill is likely to be reduced, and ewe facial expression will indicate sickness or discomfort (McLennan et al, 2016).

Examination of the udder may reveal changes to the udder and its secretion. Changes to the milk range from clots within the milk, through a straw-coloured serous liquid, to blood tinged or purulent. The udder is likely to be warm or hot to the touch. Affected areas will feel hard. These may range from a small segment to the entire half. The affected udder half may be reddened, and there may be oedema of the udder half and the surrounding skin. The supramammary lymph node on the affected side is usually enlarged.
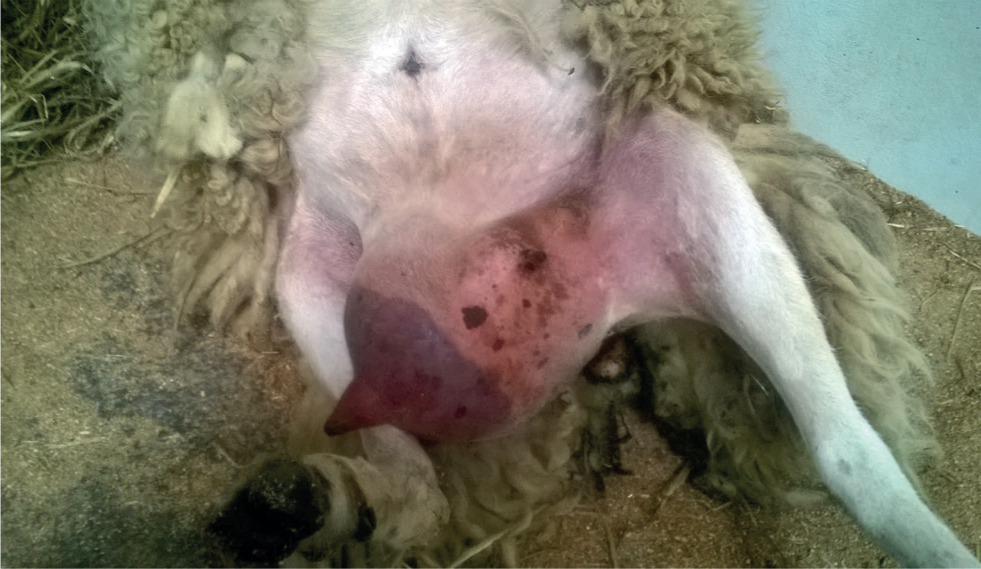
In gangrenous mastitis the affected areas change colour from red, to purple to black (Figure 3). They become cool and then cold to the touch and may weep serous fluid from the skin. As the affected tissue necroses (in sheep that survive the initial phase) separation occurs along the line between affected and healthy skin. The area of sloughing may include the mammary vein if thrombosis has occurred. The sloughing tissue often forms into a ball connected to the body by a narrow neck. This contains blood vessels, and is relatively friable, so great caution must be exercised if considering ligating and amputating this.
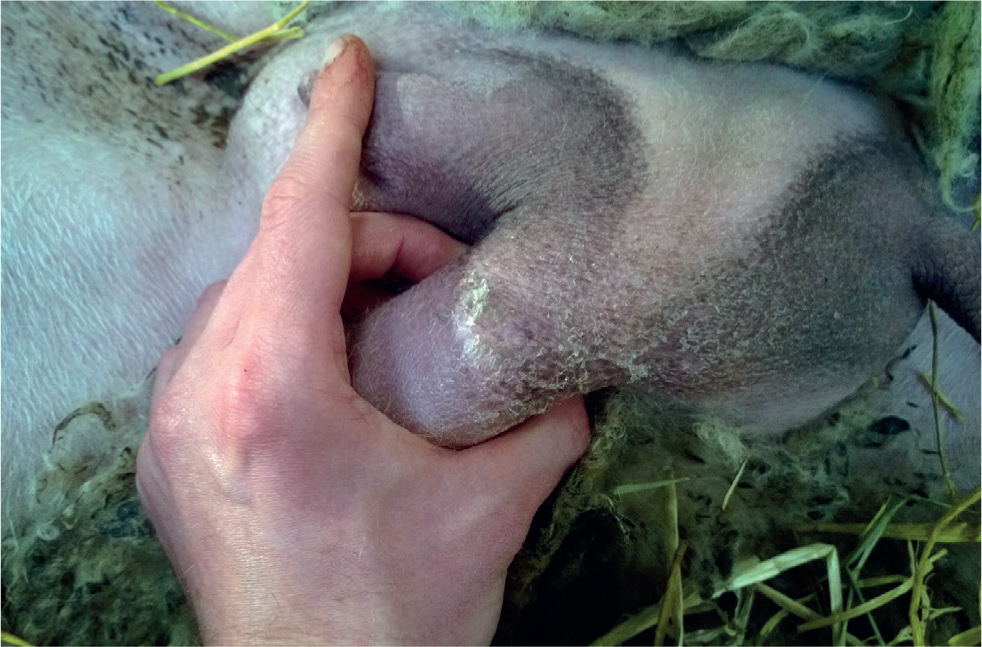
Many historical cases of mastitis are detected only at weaning. Abscessation may be detected by enlarged and irregular mammary tissue, discharging tracts may be present. Scarring may also be palpable. The teat may feel as though there is a firm cord within it.
Previous cases of mastitis may be detected at lambing as udder halves with reduced or absent production. Scarring, abscessation and ‘teat cords’ may also be felt.
Further diagnostics
Although the vast majority of clinical cases may be diagnosed by clinical signs alone, the California milk test (CMT) may be used to support a diagnosis when changes to the milk and udder are subtle (McDougall et al, 2001; McLaren et al, 2018). Bacterial culture and sensitivity can be useful for the investigation of outbreaks of mastitis and for advising on antimicrobial choices for future cases; however, because of the rapid progression of the disease, it is rarely useful for informing treatment choices in individual cases. Ultrasonography of the mammary gland has been used extensively in research settings and could theoretically be used to assist replacement selection and culling decisions (Barbagianni et al, 2017). In addition, ultrasonography of the supramammary lymph nodes may be a useful ancillary diagnostic for identifying cases of subclinical mastitis (Hussein et al, 2015). Ultrasonography can also prove useful for the occasional individual animal case of mammary enlargement not caused by bacterial mastitis (e.g. identification of herniation into the udder, biopsy of mammary neoplasia). However, it is difficult to envisage more widespread applications for ultrasonography in commercial lambproducing sheep farms.
Treatment
Acute mastitis in sheep requires treatment with systemic antibiotics and non-steroidal anti-inflammatory drugs (NSAIDs). In ewes showing signs of dehydration, fluid therapy, whether oral or intravenous, will be beneficial. Cases of chronic mastitis cannot be treated to restore function, and so require treatment only if this is required for the welfare of the animal.
The use of intramammary preparations for the treatment of cases of clinical mastitis in dairy sheep is recommended (Mavrogianni et al, 2011). There is little evidence supporting or contradicting their use in mastitis in meat sheep. In Sweden, intramammary tubes are a routine part of acute treatment (Phythian personal observation); special applicator tips for sheep are used. Where intramammary preparations are to be used, hygiene must be scrupulous to avoid the introduction of other potential pathogens into the teat. A whole tube should be used per affected half. In the authors' opinion, they should be an adjunct to systemic antibiotic treatment, rather than a replacement for it, and their use is not considered vital.
Animals affected by chronic mastitis should be culled. Those affected by acute mastitis are likely to require culling, unless the case is mild and treatment is instigated early. Their eartag number should be noted at the time of treatment and they should be provisionally placed on the cull list.
Severely systemically ill animals with gangrenous mastitis should be euthanased. There is a legitimate debate about the welfare of animals that have survived the acute phase, where sloughing of the udder tissue has begun. Certainly, these animals require close observation to prevent fly strike of the open wounds that occur as part of the sloughing process.
In terms of antibiotic choice, because of the thrombosis of minor blood vessels that occurs in acute mastitis, a large volume of distribution is required. The antibiotic chosen should be effective against the most common bacteria involved, which are Staphylococcus aureus and Mannheimia haemolytica (Gelasakis et al, 2015). Oxytetracycline, aminopenicillins and macrolides are all valid choices using these criteria. While potentiated sulphonamides would also be a valid choice on paper, pharmacokinetic differences limit their practical suitability for use in sheep (Batzias et al, 2005). Florfenicol may also be used, although it requires more frequent administration than in cattle (NOAH, 2021). Antimicrobial susceptibility testing of S. aureus and Escherichia coli isolates from sheep in Scotland and Norway indicated very low levels of antimicrobial resistance to all antimicrobial classes in S. aureus, while amoxycillin/clavulanate, macrolides, cephalosporins and fluoroquinolones showed least resistance in E. coli cases (Silva et al, 2020). Unfortunately, that study did not assess resistance in M. haemolytica. The UK Veterinary Antibiotic Resistance and Sales Surveillance Report 2019 reported that from 2017 to 2019, M. haemolytica isolated from sheep's respiratory tracts showed very low levels of antimicrobial resistance across nearly all antimicrobial classes, but resistance to oxytetracycline in 10 out of 29 submissions (Veterinary Medicines Directorate, 2020). However, given the very small sample size and biased study population (clinical samples submitted to diagnostic laboratories) in that report, those figures should be interpreted with extreme caution.
In the authors' clinical experience, macrolides or a 7-day course of penicillin/dihydrostreptomycin or amoxicillin seem to have the highest success rate. The effectiveness and ease of use of macrolides (those licensed in sheep are a single long-acting injection) must be balanced against their designation by some authorities as critically important antibiotics. (For this reason, penicillin is the drug of first choice in Scandinavia).
Given the individual animal nature of mastitis in meat sheep, the use of macrolide antibiotics, in the authors' clinical experience, is justified to treat these cases.
It is important to note that in the UK only tilmicosin, oxytetracyline and enrofloxacin have licenses specifically for treating mastitis in sheep; however, procaine penicillin, penicillin/streptomycin, penicillin/neomycin, ampicillin and amoxycillin are licensed for treating sensitive infections in sheep. Tulathromycin, gamithromycin and florfenicol are licensed for other uses in sheep and could be used under the cascade if required (NOAH, 2021). The lack of a licensed NSAID in sheep necessitates off-license use under the cascade, with the authors preferring flunixin or meloxicam (licensed in Australia and New Zealand at 1 mg/kg subcutaneously, i.e. twice the cattle dose (Boehringer Ingelheim, 2016)).
Aetiopathogenesis
Mastitis is defined as inflammation of the udder, which in meat-producing sheep, is principally caused by intramammary bacterial infection. Mastitis may also present as a component of systemic infections, notably by Mycoplsama agalactiae and small ruminant lentivirus (maedi-visna). These infections are beyond the scope of this article but the reader is referred to: Corrales et al (2007) for a review of contagious agalactia (thankfully not currently present in the UK); van der Molen and Houwers (1987) for a description of the pathogenesis of the indurative (firm) mastitis caused by small ruminant lentivirus infection; and Arsenault et al (2003) for an investigation of the impact of small ruminant lentivirus infection on lamb production in Quebec, Canada.
In meat producing systems, the most common causative organisms of mastitis in sheep are Staphylococcus spp. and M. haemolytica (reviewed by Gelasakis et al, 2015). Staphylococci have been isolated from a range of anatomic locations in clinically healthy sheep (including udder skin) and may express a variety of virulence factors leading to both clinical mastitis (principally S. aureus) and subclinical mastitis (principally coagulase-negative staphylocci) (reviewed by Vasileiou et al, 2019a). M. haemolytica is a commensal of the tonsils of lambs, from where it is transferred to the ewes' teats, with only a low infectious dose required to institute clinical mastitis (Scott and Jones, 1998). A broad array of other bacterial species has also been isolated from cases of mastitis in sheep, the majority of which are presumed to be secondary to environmental contamination (reviewed by Gelasakis et al, 2015). The authors have also experienced significant problems with outbreaks of severe mastitis caused by E. coli (Phythian personal observation).
In order to cause mastitis, these bacteria must first gain entry into the mammary gland through the teat, which forms the first line of defence against mastitis. The teat orifice is closed by local musculature, with lipid secretions onto the keratinised surface of the teat canal acting as a physical barrier to trap bacteria, which are then flushed away with the next outward flow of milk (Nickerson, 2002). In addition, lymphoid follicles present at the junction between the teat canal and the cisterns are thought to provide additional protection against deeper invasion by bacteria (Mavrogianni et al, 2005; Fragkou et al, 2007). Factors affecting teat function, in addition to innate and adaptive immune responses to bacteria that penetrate into the mammary gland, are likely to affect the probability of ewes developing mastitis.
Risk factors
Factors affecting ewe defences
The teat is the first line of defence against mastitis, and as described above, flushing of the teat canal is important in preventing bacteria ascending into the udder. Where milk supply is inadequate, unproductive suckling by lambs has the potential to facilitate bacterial contamination, in addition to inducing traumatic damage to the teats (Cooper et al, 2013), analogous to a poorly maintained milking parlour in dairy systems (Manning et al, 2021). Inadequate nutrition is a clear risk factor for this (Arsenault et al, 2008; Huntley et al, 2012; Onnasch et al, 2002), with ewes underfed protein during pregnancy having approximately four times greater odds of suffering acute mastitis (Grant et al, 2016). Large litter size is also a risk factor for mastitis (Cooper et al, 2016), with Waage et al (2008) suggesting that large litters are a more significant risk factor in younger ewes, presumably because of decreased milk yield. Pregnancy toxaemia has also been shown to increase susceptibility to M. haemolytica mastitis (Barbagianni et al, 2015). Concurrent chronic disease or endoparasitism may have similar effects, although it is unclear to what extent effects are related to reduced milk supply versus reduced immune function. Dystocia has also been reported to increase the risk of mastitis, although the underlying mechanism is unclear (Waage and Vatn, 2008).
The protective effect of the teat may also be affected by environmental conditions. To the authors' knowledge there are no studies utilising climatic data to predict mastitis risk in meat producing sheep (as has been performed for dairy sheep (Giannakopoulos et al, 2019)). However, mastitis is anecdotally associated with cold weather and high winds, possibly as a result of chapping of the teat, although increased metabolic demands during such weather periods, and poaching of areas of shelter have also been suggested as potential causes.
As discussed above, lymphoid follicles at the proximal end of the teat canal also have an important role in preventing bacterial colonisation of the udder (Mavrogianni et al, 2005; Fragkou et al, 2007). Research shows that these follicles are reduced in size in ewes affected by orf, potentially explaining higher rates of mastitis in affected ewes (in addition to cross-suckling) (Mavrogianni et al, 2006).
Once bacteria have infected the udder the ewe's immune defences are necessary to try to control infection, and genetic variation in this immune function may explain breed variation susceptibility to mastitis (Torres-Hernandez and Hohenboken, 1979; Watson et al, 1990, Larsgard and Vaabenoe, 1993; Waage and Vatn, 2008). Episodes of previous mastitis within a lactation have been shown to increase the odds of a second case of mastitis within a season 17-fold (Arsenault et al, 2008). Further, the presence of palpable intramammary masses during both pregnancy and lactation have been associated with future intramammary masses, and marginally reduced lamb growth rates (Grant et al, 2016). It is uncertain whether this is a result of unresolved infection, scar tissue or individual susceptibility; however, it supports the common advice to cull ewes after a case of mastitis, and to check udders thoroughly before making breeding decisions.
Factors increasing contagious transmission
Cross-suckling is anecdotally associated with the contagious transmission of mastitis pathogens and while empirical data are lacking, it is somewhat intuitive that a lamb unable to feed at its dam because of poor milk supply, foul milk, or a painful udder will seek milk elsewhere and potentially transfer pathogens to other ewes. Painful orf lesions may also have a similar effect, in addition to more direct effects of orf infection (see above). It is therefore sensible to isolate ewes with clinical mastitis if practical: this also allows better monitoring of treatment and assessment of whether lambs need to be removed from the ewe.
Where stripping of the udder is performed, milk should be collected in a container, rather than being stripped onto the bedding, to reduce contamination of the environment with mastitis pathogens. Staphylococci may also be resident on human skin, therefore clean gloves should be worn for this procedure, to reduce the risk of iatrogenic transmission.
In addition to reducing faecal contamination of the udder, dagging and other fly control methods may also reduce transmission of mastitis pathogens, although the significance of this mode of transmission is uncertain.
Factors increasing environmental contamination
Factors affecting lambing environment hygiene and contamination of the udder are likely to affect the prevalence of environmental bacterial mastitis. If weather conditions permit, lambing outside is associated with a lower risk of mastitis than lambing indoors (Cooper et al, 2016). Where indoor lambing is necessary daily bedding addition has been shown to be associated with a lower risk of mastitis than with less frequent addition (Cooper et al, 2016). The degree of drainage below the bedding is also of importance with hardcore floor sheds linked to approximately 1.6 times lower risks of mastitis than concrete or earth floors (Cooper et al, 2016). Hygiene pre-housing may also be a significant factor — the authors have experienced cases putatively linked to grazing poached pastures and root crop fields prior to housing, for example. Dagging can also help to reduce faecal contamination of the udder and may therefore reduce risks of Enterobacteriaceae (e.g. E. coli) mastitis.
Udder conformation may also affect the likelihood of environmental contamination of the udder. This is presumably responsible for the higher somatic cell counts in ewes with pendulous udders observed by Huntley et al (2012) at 14 days post-lambing. That study utilised udder conformation scoring, which has been shown to be heritable in dairy sheep (Casu et al, 2006), and Crump et al (2019) showed that Texel udder conformation traits assessed at 7–14 weeks post-lambing were heritable. Further, McLaren et al (2018) showed that somatic cell counts were correlated with udder conformation assessed in mid lactation and Grant et al (2016) showed ewes with forward-pointing or downward-pointing teats to have 2.5 and 4.7 times greater odds (respectively) of developing acute mastitis. Teats placed at 4–5 and 7–8 o'clock positions have the lowest risk of mastitis.
Prevention
Prevention of mastitis focuses on reducing the risk factors outlined above. Ewes should be in adequate body condition at lambing (target 3.0–3.5 for lowland ewes) and nutrition in the final 4 weeks before lambing should provide adequate levels of protein and energy. This may be assessed through metabolic profile (specifically measuring beta-hydroxybutyrate, blood urea nitrogen and albumin levels) (Phillips et al, 2014). At the point of lambing, assessment of the colostrum quality using a refractometer and udder evaluation can also assess pre lambing nutrition levels and identify potential risks for mastitis.
Ensuring that energy and protein requirements are met during the lactation period is important.
Where sward heights become too short, supplementary feeding (which may be concentrate, forage or both) is required. Prevention of selenium deficiency may also aide in the good immune function of the udder — although this has not been proven in sheep, it is well documented in dairy cattle (reviewed by O'Rourke, 2009). Where orf has been a problem in previous years, then vaccination of ewes may be attempted. The timing of this is key, as less than 7 weeks before lambing can result in the shedding of viral contaminated scabs into the housed environment. Administration should occur in the axilla, as per the data sheet; vaccination in the groin increases the risk of transmission to lambs.
Any cases of mastitis should be marked for culling. At weaning, all udders should be palpated, and those with lumps (presumably because of chronic abscessation or scar tissue within the mammary gland), or scar tissue within the teats should be culled. This should be repeated prior to tupping, when selecting ewes for mating groups.
Recent research on udder conformation and teat position suggests that there may be benefit in culling ewes on these criteria in preventing mastitis. Increasing the levels of recording on farm with reference to mastitis cases and associated characteristics such as parity, teat position, breeding lines, body condition score and prelambing nutrition including forage analysis, will help to build a prevention control plan for the following lambing period.
There is some evidence from dairy sheep suggesting that treatment with intramammary antibiotics at dry off (dry period therapy) is beneficial in reducing the incidence of mastitis in the following lactation (reviewed in Petridis and Fthenakis 2014). In suckled flocks:
- Hendy at al (1981) found a reduction in mastitis between dry-off and tupping (4.5% controls vs 1.5% treated), through blanket use of a cow dry-period intramammary preparation
- Hueston et al (1989) found untreated ewes were 2.6 times more likely to have culture positive milk samples taken at 1–3 weeks of lactation, than ewes treated at dry-off with a cow dry-period preparation.
Consideration needs to be given to the different lengths of dry period in these systems, and the greater importance of M. haemolytica in meat sheep mastitis, compared with dairy ewes, the number needed to treat (NNT) based on the available evidence, and responsible antibiotic usage, when deciding whether to advise use of intramammary antibiotics at dry-off in meat sheep flocks.
There is a hereditary component to susceptibility to mastitis and to high somatic cell count (McLaren et al, 2018). Crump et al (2019) estimated the heritability of intramammary masses as 0.18 and teat lesions as 0.17; the same study found higher heritability for phenotypic characteristics considered risk factors for mastitis such as teat placement and teat length (heritability of 0.35 and 0.42, respectively). There have been attempts to reduce mastitis incidence through selective breeding in Texel sheep.
An S. aureus vaccine (Vimco, Hipra) has been developed for use in dairy sheep and goats for the reduction in the incidence and severity of mastitis associated with S. aureus and coagulase-negative staphylococci. In dairy sheep systems use of the vaccine resulted in approximately a 50% reduction in incidence (Vasileiou et al, 2019b) and severity of the remaining cases. Data on the vaccine use are limited. Case reports from Northumberland (Henry, 2018), suggested that a similar reduction in incidence occurred in two meat sheep flocks where other changes had failed to bring a clinical mastitis problem under control. Henry suggested in his report that the vaccine would be cost effective if the incidence rate is above 10%.
Improvement of the environment, e.g. more frequent bedding up, ensuring the shed is well drained, clean straw, or avoidance of densely stocked and muddy pastures outdoors, will also help prevent cases.
Conclusion
Mastitis is a major cost to the meat sheep industry, but the impact may be underestimated if only acute, treated cases are considered. There is a myriad of further costs, both from clinical cases, but also from chronic cases and subclinical cases.
Acute cases, for which veterinary advice or treatment are sought, are an opportunity to discuss the wider impact of ovine mastitis on a client's flock. This can also facilitate an exploration of the pertinent risk factors for that flock, thereby allowing the development of a tailored preventative approach.
KEY POINTS
- Mastitis is ewes is a common clinical scenario in meat flocks across the UK.
- Veterinary involvement can lead to improved vet-farmer engagement and more detailed flock health discussions.
- Acute and chronic mastitis cases lead to reduced ewe longevity in the flock with welfare, production and financial impacts.
- The most common bacterial pathogens involved are Mannheimia haemolytica and Staphylococcus species.
- A significant risk factor for mastitis relates to impairment of the udder defences, including teat damage due to over suckling when milk supply is inadequate.


