Malignant catarrhal fever (MCF) is caused by Gammaherpesviruses of the Macavirus genus, namely ovine herpesvirus 2 (OvHV-2), caprine herpesvirus 2 (CpHV-2) and alcelaphine herpesvirus 1 (AlHV-1) (Russell et al, 2009). In the USA, Canada and Europe (including the UK) the sheep-associated (SA-MCF) form caused by OvHV-2 appears most commonly and has been documented in more than 30 species; however those of most interest to the UK are cattle, bison, water buffalo, deer and pigs (Flach et al, 2002; Russell et al, 2009; Li et al, 2014; Amin, 2015).
Sheep are considered the main reservoir host, and until recently were considered to be clinically unaffected. While this generally remains the case, there have been a small number of reports where sheep have died and been found to demonstrate pathologies similar to those found in other species affected by MCF in the absence of other causes of disease (Pesavento et al, 2019). An MCF-like syndrome can also be generated experimentally when sheep are exposed to large doses of OvHV-2 (O'Toole and Li, 2014).
Characteristic of herpesviruses, OvHV-2 can exist as a lytic infection – actively producing new virus particles, or a latent infection – a dormant transcriptionally and translationally suppressed state. Latent OvHV-2 infections are found in both reservoir and clinically susceptible hosts, with work suggesting a large proportion of cattle can carry the virus subclinically (Amin, 2015). Subclinical infection has also been demonstrated in bison (Angell et al, 2023). This quiescent form of infection adds to the challenge of controlling OvHV-2, as animals remain persistently infected with the potential for the virus to recrudesce at any point, leading to shedding of virus and subsequent disease.
In cattle, following infection with OvHV-2, usually from a sheep source, there is an incubation period ranging from weeks to months, with an approximate average incubation period of 50 days (Li et al, 2014). In cattle, clinical case numbers tend to be low, with usually only one or two animals affected during an outbreak. However, in bison and buffalo, much larger proportions of the exposed group may be affected. For example, in 2003, a SA-MCF outbreak occurred on a bison feedlot in Idaho, USA. This outbreak was considered to be as a result of exposure to sheep for 19 days and resulted in a total of 825 deaths out of the 1610 bison exposed (51.2%), with deaths peaking at a rate of 41 head per day (Li et al, 2006).
Herpesvirus virion
Mature herpes virions vary from 120–260 nm in size. The core of the virion comprises double-stranded DNA held inside a 115–130 nm icosahedral capsid. The nucleocapsid is surrounded by a dense proteinaceous matrix called the tegument which is enclosed in a bilipid membrane containing viral glycoproteins (Sadeghipour and Mathias, 2017). Herpes virus entry and fusion to the host cell requires three of these viral glycoproteins ubiquitous in all herpesviruses, gB and heterodimer gH/gL (Eisenberg et al, 2012). On cell entry, herpesvirus will undergo one of two pathways. In the latent form of infection, the viral DNA will form a circular episome and remain in the nucleus where it can lie dormant for years. Latent mRNAs are transcribed which prevent production of new virions and cell death. To remain latent during host cell replication, DNA episomes tether themselves to a host chromosome to be divided into each daughter cell (Cohen, 2020). In lytic or virulent infection, viral DNA is replicated in the host nucleus. Tegument protein VP16 activates transcription of the first set of viral genes ‘immediate early genes’. These are translated by ribosomes into proteins which in turn activate transcription of ‘early genes’. Early genes are translated into viral polymerases which commence replication of the viral DNA. New viral DNA expresses ‘late mRNA’ which is translated into capsid and envelope proteins in free ribosomes and on the endoplasmic reticulum respectively. Capsid proteins enter the nucleus to encapsulate viral DNA, which then migrates through the nuclear membrane, through the endoplasmic reticulum where the envelope forms, through the Golgi apparatus and is egressed from the cell (Sadeghipour and Mathias, 2017). While most genes code for structures of the OvHV-2 virion, some have functions in host immunomodulation including MHC class II signal disruption and production of their own viral cytokines to modulate inflammation (Zuo and Rowe, 2012; Ouyang et al, 2014).
Clinical signs associated with SA-MCF
Following infection with OvHV-2, the animal develops an inflammatory response with dysregulation of cytotoxic T-cells, predominantly in blood vessels and associated mucosal surfaces. As such, the clinical signs of SA-MCF can vary considerably depending on which blood vessels and mucosal surfaces are affected (Russell et al, 2009; Li et al, 2014; Headley et al, 2020). The head and eye form is most frequently seen in cattle (Russell et al, 2009; Li et al, 2014; O'Toole and Li, 2014) with some or all of the following clinical signs:
- Marked pyrexia (>40°C)
- Mucoid/mucopurulent/haemorrhagic nasal discharge (Figure 1)
- Ulceration of the nose/muzzle/buccal cavity
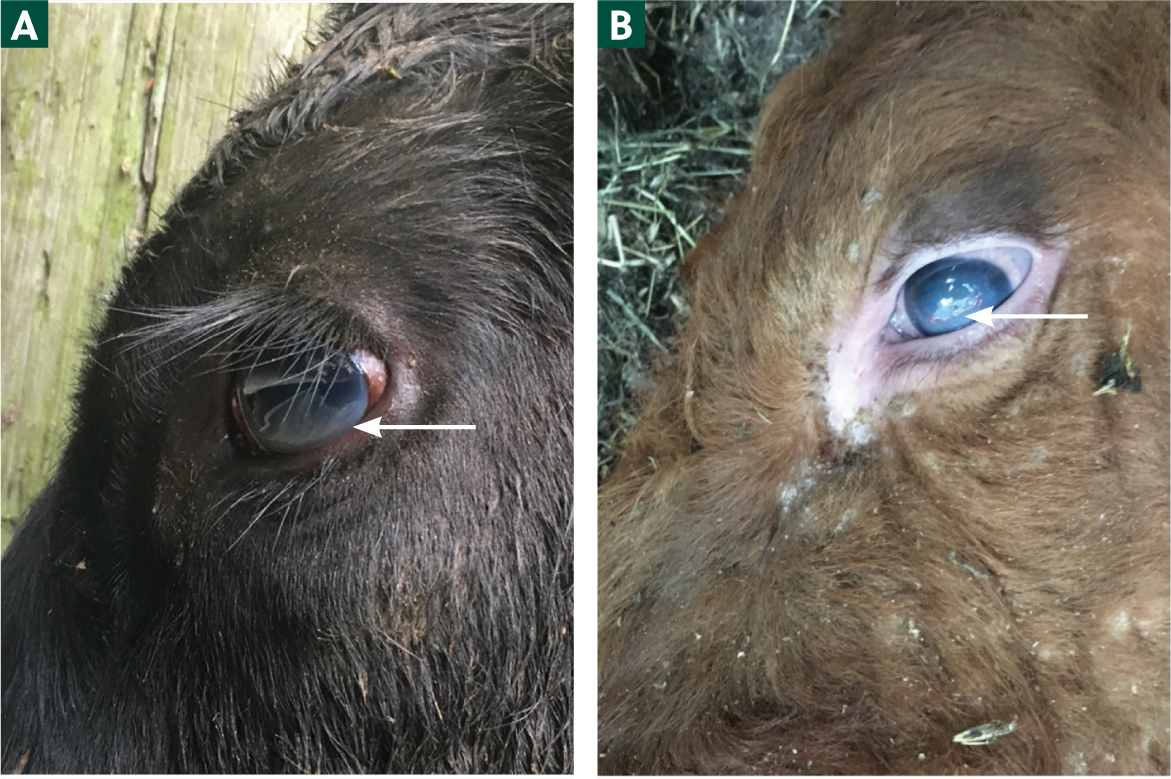
- Increased lacrimation (Figure 2)
- Conjunctivitis
- Severe keratitis/corneal oedema (Figures 3a and 3b), hypopyon and partial/complete blindness.
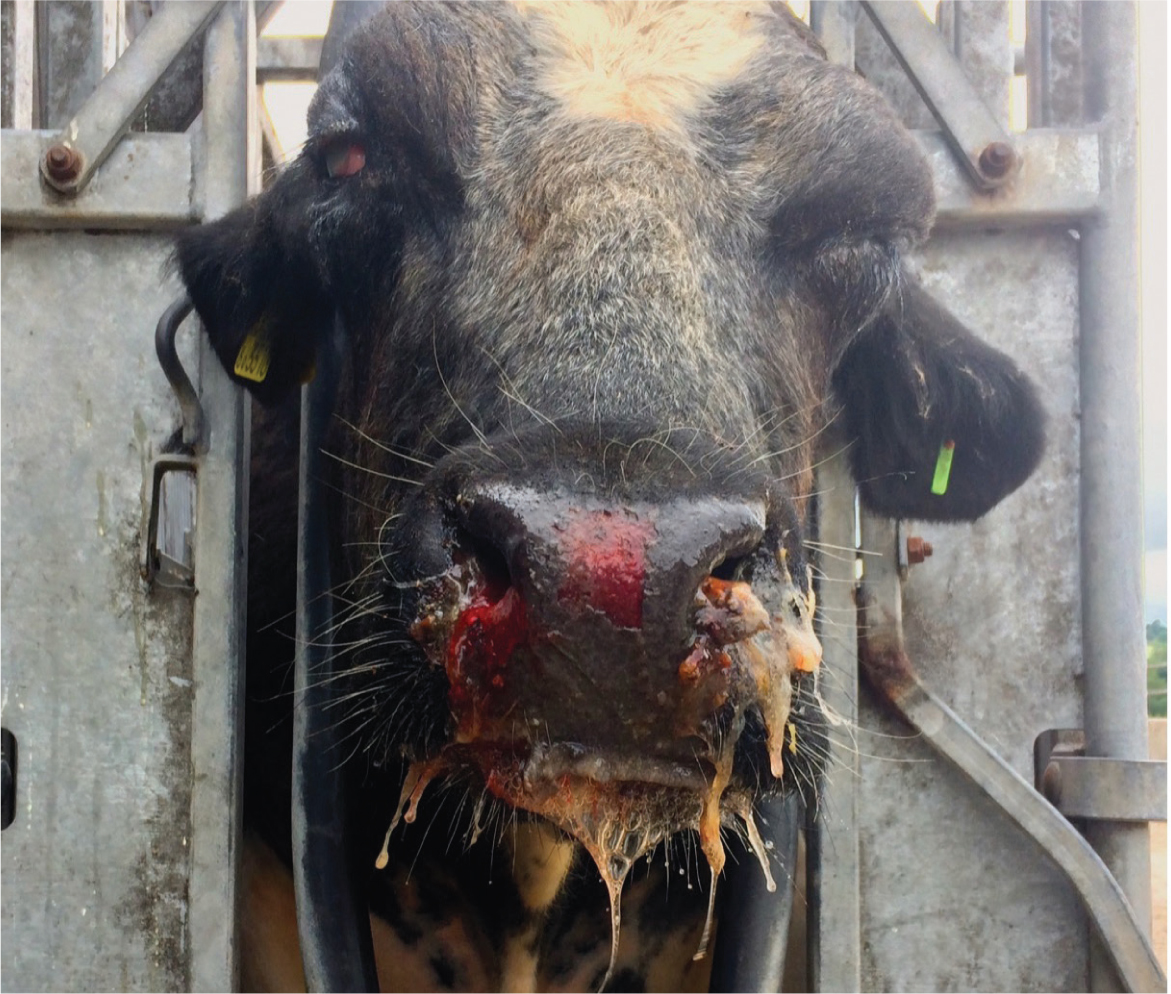

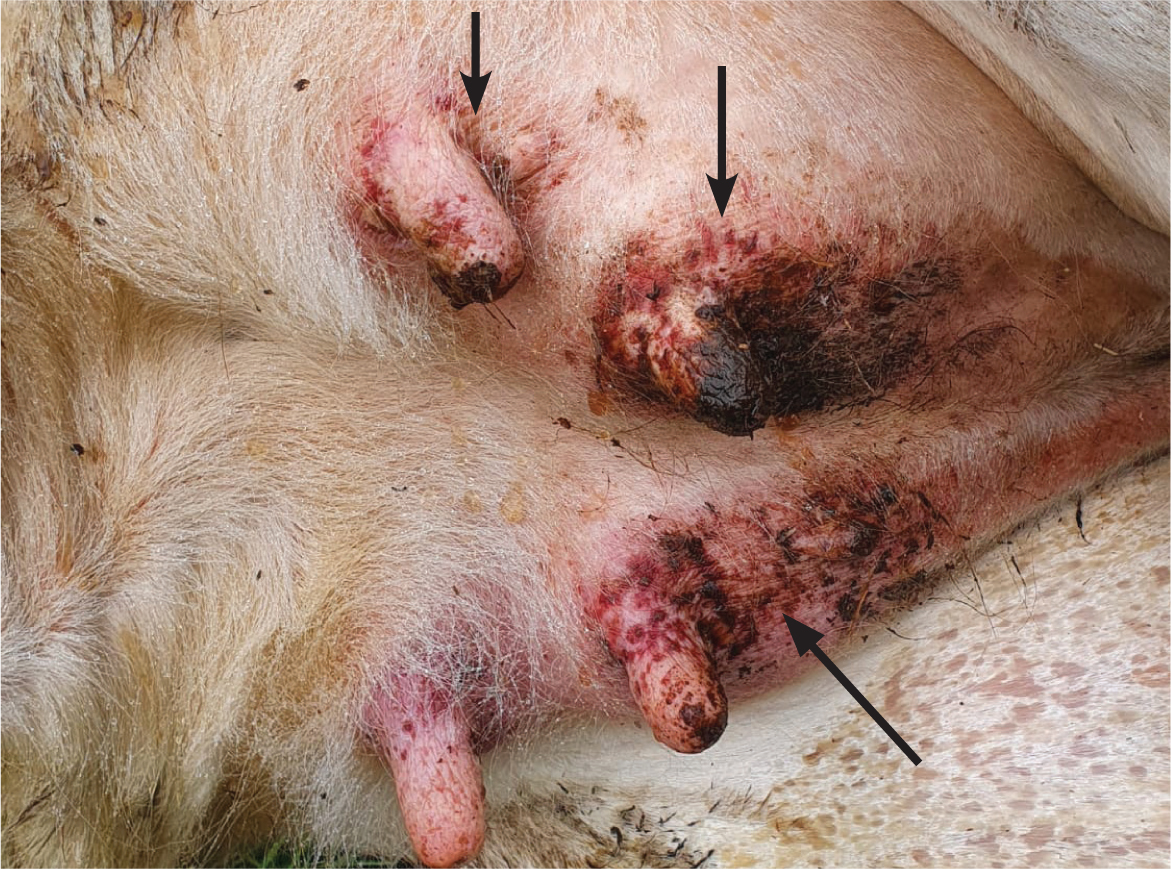
Diarrhoea, dermatitis (Figure 4) and neurological signs may also be present. In bison and buffalo, the course of the disease may be far more rapid, sometimes simply presenting as sudden death (O'Toole and Li, 2014).
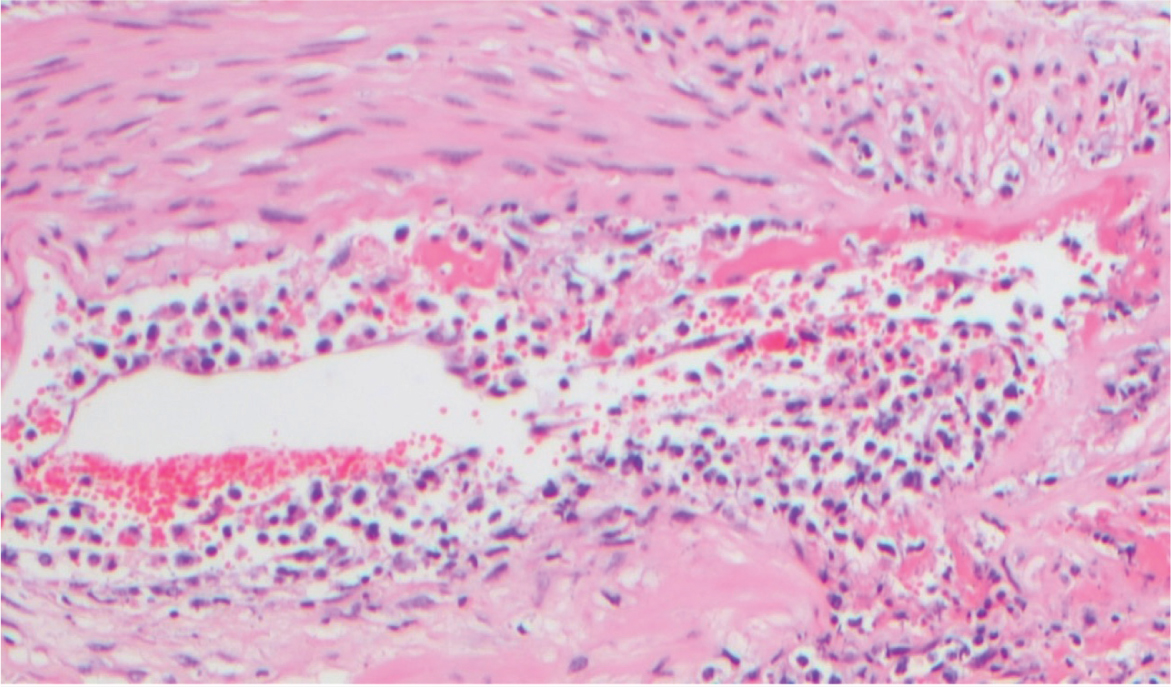
At post-mortem, in addition to the lesions seen in the live animal, a local or generalised lymphadenopathy may be observed, and haemorrhages may be noted in the nasal mucosa, buccal mucosa, throughout the gastrointestinal tract, within the respiratory system and in the urinary bladder. Indeed, in bison, pathology in the kidneys and urinary bladder seems to be a frequently consistent feature (O'Toole and Li, 2014). Histopathologically, a non-suppurative lymphoproliferative vasculitis is seen in affected tissues (Figure 5).
Pathogenesis
As the reservoir host, new-born lambs are nearly always born free of infection, but generally become infected during the first year of life, with lambs aged 6–9 months potentially more likely to be shedding virus from nasal secretions, and at a greater concentration compared with older sheep (O'Toole and Li, 2014). Infection in susceptible hosts is usually via aerosol, with reports of susceptible hosts developing clinical signs at a considerable distance away from the source host, up to at least 5 km (Li et al, 2008) (Figure 6).
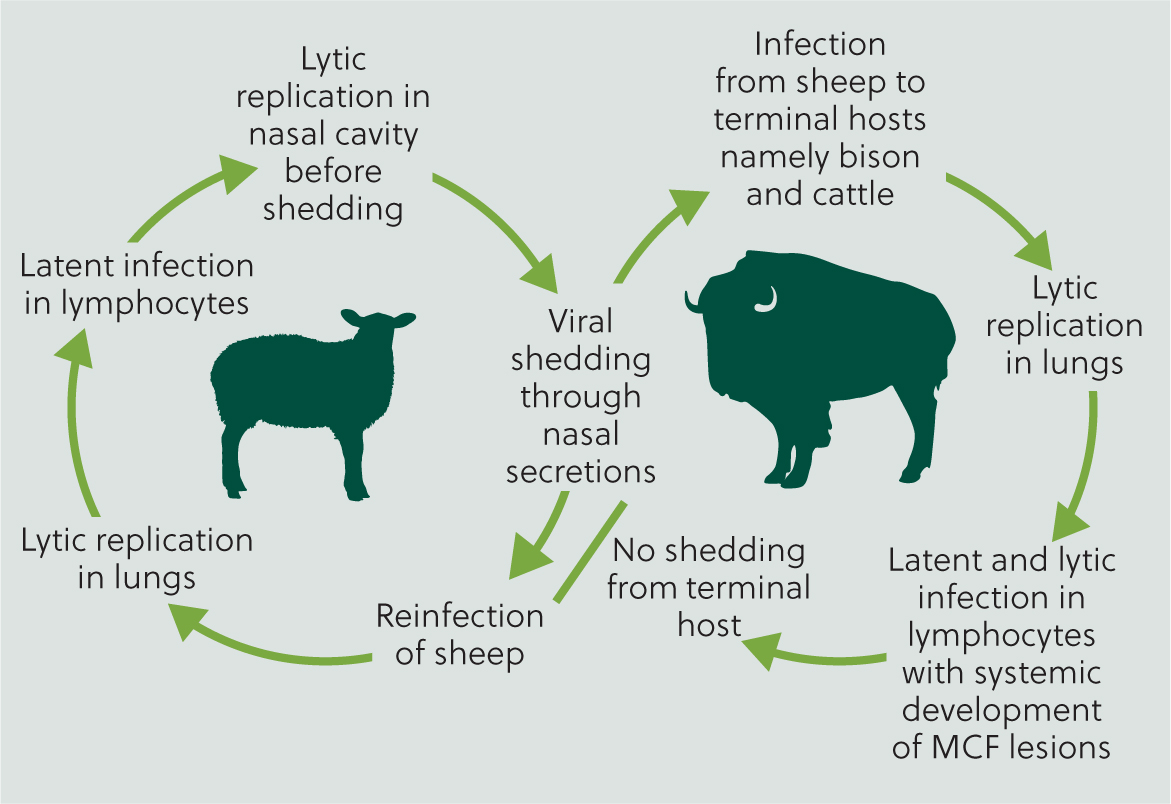
In a susceptible host, following infection, initial viral replication is usually in the respiratory tract, and at some point in cases where clinical disease is observed, lymphocytes become infected (Russell et al, 2009). Experimentally, T-cell hyperplasia, proliferation and then infiltration of vascular endothelial and smooth muscle cells is described, with a resultant arteritis (Buxton et al, 1984; Saura-Martinez et al, 2021). Microarray of host immune gene transcripts in lymph nodes of MCF-infected cattle, compared to healthy animals, reveal a pro-inflammatory profile of chemokine expression, notably depletion of interleukin-2 (Meier-Trummer et al, 2009). Research has demonstrated a specific role for infected cytotoxic T-lymphocytes in the inflammatory processes observed, alongside monocytes and locally proliferating macrophages. In addition, vascular endothelial cells, medial smooth muscle cells and adventitial fibroblasts may all be subsequently infected (Saura-Martinez et al, 2021). The dysregulation of the cytotoxic T-cells results in tissue destruction and necrosis, resulting in the severe pathologies seen (Buxton et al, 1984; Schock and Reid, 1996; Saura-Martinez et al, 2021).
Where recovery occurs, the lymphoid arteritis resolves, although a chronic arteriopathy may be observed histologically with the development of a generalised chronic obliterative arteriosclerosis (O'Toole et al, 1997).
Diagnosis
A diagnosis of SA-MCF may be suspected based on the history and clinical signs. However, virus detection, particularly at high concentration, alongside characteristic gross post-mortem findings and histopathology of affected tissues, are necessary for confirmation.
Peripheral blood leukocyte OvHV-2 copies remain stable despite fluctuation in OvHV-2 copies found in ovine nasal samples, indicating that use of blood sampling for diagnostics to identify infective reservoir sheep and shedding events may not be accurate (Li et al, 2004). Key differential diagnoses to exclude are mucosal disease (uncommon form of disease caused by bovine diarrhoea virus, and infectious bovine rhinotracheitis).
Treatment
Treatment tends to be empirical, focusing on suppressing or reducing inflammation using steroids or non-steroidal anti-inflammatory drugs (NSAIDs), antibiosis to treat expected secondary opportunistic pathogens, and good nursing care, including other symptomatic treatments. Clinically affected animals exhibiting head and eye form signs may struggle to see well enough to find food and water and may collide with their surroundings (Figure 1). They may also have difficulty eating with severe nasal or buccal lesions. Caring for them within a confined space, ensuring it is free from possible causes of injury, can make it easier for them to find food and water, together with symptomatic treatment. Only very few diseased animals survive, and some of those that do may continue to show fulminating clinical signs of varying severity. As herpes viruses become latent, recrudescence may lead to a recurrence of clinical signs and further viral excretion. Clinically affected animals may also excrete virus and be a risk to further members of the herd (O'Toole et al, 1997), so strict biosecurity is important to manage this risk.
Recovery from SA-MCF is possible, but uncommon (O'Toole et al, 1997; Munday et al, 2008; Angell et al, 2023). However, while animals can survive, chronic changes including persistent corneal and scleral changes, as well as a chronic arteriopathy are found at post-mortem, and so animals may need to be slaughtered below optimum targets. Early culling should be considered because of the expectation of reduced performance outcomes (eg meat and milk), and the possibility of recrudescence, or increased susceptibility to other diseases.
Prevention
Sheep are considered the main reservoir host, and those less than 1 year of age are considered more likely to shed greater volumes of virus compared to adult animals. Transmission is usually via aerosol, although could be via fomites or direct nose-to-nose contact. Clinical case reports have documented cases developing 5 km from the nearest sheep source and, therefore, grazing and management practices with cattle and sheep in close contact can increase the likelihood of transmission. Therefore, considering all these factors, where possible, managing valuable animals away from high-risk groups of sheep is important, with greater distances reducing the risk further.
Additionally, given the infectious cause, anything that can impair the health of the susceptible end hosts could increase their susceptibility. Optimising nutritional status, parasite control and prevention of other infectious diseases is likely to increase the resilience of animals to infection.
Currently there is no commercially available vaccine against OvHV-2 for end host species, although an experimental recombinant vaccine has been used with limited success in bison in the UK (Angell et al, 2023). A vaccine against Wildebeest-associated MCF (WA-MCF) exists for use in Africa (Cook et al, 2019) and consideration has been given to the possibility of using this for cattle and bison against SA-MCF. However, neutralising antibodies have been shown to be host- and pathogenx-specific (Taus et al, 2015), and therefore cross protection from this WA-MCF vaccine against SA-MCF is considered unlikely (Cook et al, 2019).
Conclusions
SA-MCF remains a devastating disease for affected farms and infected individuals because of the lack of effective treatments and preventive measures. The only real preventive measure is to increase the distance between susceptible hosts and sheep, which in many areas is impractical or impossible. Work on the pathogenesis of the disease may facilitate the development of new treatment options and open up possibilities for vaccine development. With regard to animals that survive, early culling is recommended owing to the ongoing consequences of chronic arteriopathy, the possibility of virus recrudescence and an increased susceptibility to other diseases.
KEY POINTS
- Sheep-associated malignant catarrhal fever (SA-MCF) can affect many host species, although some are more susceptible than others. It produces severe pathology, is difficult to manage and frequently fatal.
- The disease is characterised by a severe vasculitis in affected tissues, with dysregulation of cytotoxic T-lymphocytes resulting in tissue destruction and necrosis.
- Diagnosis of SA-MCF can be suspected based on the clinical signs and history, but virus detection and histopathology are needed for a definitive diagnosis.
- Prevention of SA-MCF in susceptible hosts should focus on optimising overall health to increase host resilience, and where possible maintaining as great a distance as possible between susceptible hosts and sheep, particularly growing lambs.
- Animals with SA-MCF may survive, but a chronic arteriopathy will remain. These individuals should be culled early to avoid virus recrudescence and vulnerability to other diseases, as well as to realise some value from the animal.


